AS INCLUSIONS FOR ITS CLINICAL TRIAL FOR A NEW LASSA FEVER TREATMENT PROGRESS, THE INTEGRATE CONSORTIUM CONTINUES TO STRENGTHEN STAFF CAPACITY IN KEY REGIONS TO ENSURE SAFE AND EFFECTIVE RESEARCH IMPLEMENTATION.
Lassa fever causes an estimated 300,000 – 500,000 deaths yearly in West Africa. Between January and August 2025, the Nigeria Center for Disease Control (NCDC) reported 854 confirmed cases of Lassa fever of which 159 people have died; a clear indication of the urgent need for effective treatment.
As the INTEGRATE consortium implements the first clinical trial in West Africa to identify new and more effective drugs to treat Lassa fever, capacity building for sustainable clinical research is ongoing through technology transfer and training in the region. The priority objective of the consortium is to set up the ISTH-ANRS 0409s INTEGRATE study: a multicentre, phase II-III, comparative, controlled, randomized, superiority trial in open-label parallel arms. The trial starts off at the Federal Medical Center Owo (FMCO) Ondo state and the Irrua Specialist Teaching Hospital (ISTH), Edo state, Nigeria, two of the world’s largest Lassa fever treatment centers. The preparation process for the trial has been finalized, and inclusions have begun at the FMCO site.
Site Initiation Training for key staff at FMCO and ISTH, is a key component of the start of clinical trial inclusions.
In February and July 2025, doctors, nurses, pharmacists, social scientists, clinical investigators, data managers and other key professionals at the forefront fighting against Lassa fever in Owo and Irrua respectively, were taken through an intensive multiple week training to equip them with the necessary knowledge and skills to effectively manage INTEGRATE trial participants.
Speaking after the training session at Irrua, Dr Cyril Erameh, Site Principal Investigator (PI) for the INTEGRATE study at ISTH, emphasized that trials need valid results and must prioritize the safety of patients. He highlighted the need for a wealth of experience to ensure proper implementation and valid results.
“This Site Initiation Training conducted by the sponsors and coordinating partners of the clinical trial is very important to re-train us on protocol related issues. This training particularly addresses how to handle adverse events, protocol deviations, how clinical management is done and other parts of implementing the trial.
The INTEGRATE consortium partners in the global north share capacity from their own years of experience in clinical trials to the global south to improve the implementation of clinical trials in Africa, particularly, West Africa.”


Dr Ihemekale Isaac, the lead case manager for infectious and emergent diseases with the Federal Medical Center of Owo (FMCO) who plays a critical role in facilitating the patient inclusions, also added:
“The site opening training was an opportunity to go through all stages of the clinical trial with the key staff engaged in the process and answer all their questions and ensure that the inclusions are safely done. I am confident that we are all prepared for the trial. One of the key areas is the consenting process; when we explain to the patient in detail what the trial entails, it lets them follow their normal reasoning that allows them to make an informed decision to participate in the trial. ”
Both trainings, led by the International Clinical Trial Unit comprising ALIMA, BNITM, University of Bordeaux and PACCI, were also facilitated by Dr Femi Ayodeji, Public Health Physician, Head of Infection Control and Research Center and Site PI at FMCO, and Professor Sylvanus Okogbenin, International PI at ISTH. Participants were trained in Good Clinical Practice (GCP) and ethics, on the protocol and standard operating procedures of the study, sponsorship, medical and paramedical management, monitoring, disease-related complications and biological sample management.
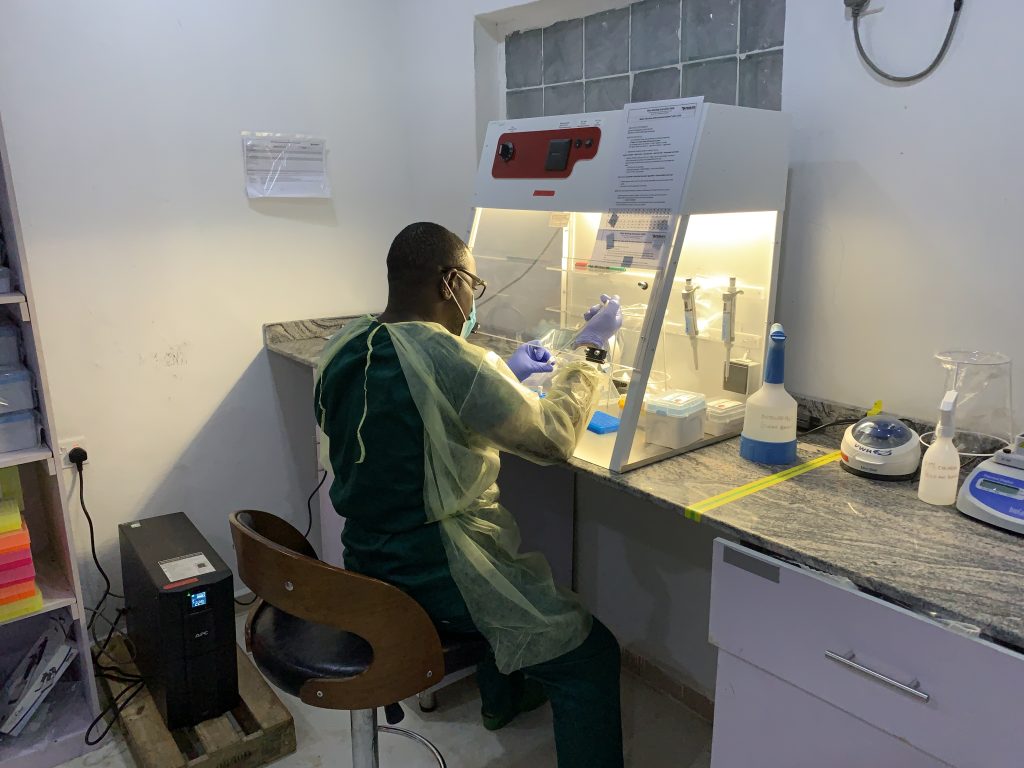
Akinola Joseph, a laboratory scientist and laboratory manager at ALIMA, said the training served as an eye opener to appreciate the importance of clinical trials in bringing lifesaving treatments to the forefront.
“I learnt the significance of the clinical trial and the overall benefit of getting an effective therapeutic product in combating the menace of Lassa Fever in Africa.”
With the training successfully completed, almost a hundred trained site personnel will carry out participant recruitment in accordance with GCP guidelines, ensuring smooth trial implementation and adherence to the research protocol and being compliant with the highest international standards. Regular monitoring visits will be conducted to assess compliance, address challenges, and provide ongoing support. Additionally, continuous capacity building and refresher training will be provided as needed to maintain high-quality standards throughout the study.
Professor Michael Ramharter, Professor for Clinical Tropical Medicine in Hamburg and Head of Department of Clinical Research at the Bernhard Nocht Institute of Tropical Medicine says improving the treatment options is key to improving patient management.
“This training is very crucial but it is multi-directional and multi-dimensional. I am an advocate that training is not a one-way road. If we look at expertise in Lassa fever patient management, FMCO and ISTH have a lot of experience and we learn from them on how to take care of patients, what are the issues, and how to address them in clinical trials. On the other hand, a lot of technical expertise for clinical research, or for bio-statistics, or for laboratory analysis is required; we bring this into the collaboration and we train scientists here. So, learning from each other, I think, is the cornerstone of the success of this project”.

In addition to the Site Initiation Visits, specific trainings on pharmacovigilance were organized in parallel to strengthen the knowledge and practices around safety monitoring and adverse event reporting. These trainings were implemented in two key phases. The first phase was conducted prior to the start of the study and was intented to the sponsor representatives and, when deemed necessary, was extended to the site PIs and clinical staff. It was mostly a theoretical training including a series of webinaire associated with practical case studies. As part of this, the International PI and the Site PI from ISTH traveled to Paris, where they met with the safety team and had the opportunity to work directly on the safety database and practice on real-life case simulations.
On this strong basis, the second phase now consists on a learning-by-doing approach with the Nigerian sponsor team. This ongoing training process aims to promote progressive autonomy by confronting them with safety-related difficulties that may be encountered throughout the course of their research. Regular safety case review meetings are currently being set up to collectively analyze adverse event reports arising from the trial, discuss causality assessments, and ensure an accurate safety reporting according to local and international regulatory requirements. This will help to the development of a sustainable pharmacovigilance system at the Nigerian sponsor level. All these training activities are in line with the INTEGRATE consortium’s long-term objective to reinforce local capacities and build a sustainable research environment in accordance with GCP guidelines.
So far, 17 patients have been included in the Clinical Trial which began in FMCO in May 2025, and is scheduled to begin in ISTH in September, 2025.
INTEGRATE is funded by the European Union’s EDCTP3 programme, with clinical trial co-sponsorship by the French national medical research agency, ANRS-MIE and the Irrua Specialist Teaching Hospital (ISTH).


